The Document Approval System (hereinafter: DJR) is an enterprise IT system unique in its kind, which makes the editing, review, approval, e-signing processes of electronic documents available to users in a simple and user-friendly manner.
The electronic signature function of DJR is available integrated into the approval process, the signing process itself is supported by the certification solution – qualified central certification system, qualified certificates, time stamping – of a qualified trust service provider. Thus the documents signed in DJR are provided with a qualified electronic signature, which is equivalent to a handwritten signature in the whole of the European Union, and has fully conclusive legal effects in Hungary.
DJR makes electronic signature technology available to users as a tool that can be used easily and routinely. It incorporates a central service that can be integrated into enterprise environments, processes, and replaces traditional (paper+pen), email or other “manually controlled” document approval, circulation and signing processes. In addition to the signing processes initiated by the users themselves on the user interface – thanks to the interfaces developed for communication – DJR also enables the development of integrations with other systems.
The participants of processes can manage their signing and review tasks on a simple and clean web interface. The documents signed in DJR can be retrieved anytime – by the users with the appropriate privileges – using the query interface of the system.
Using DJR, the last building block of process digitalization falls into place, and the electronic document generated in the given enterprise process does not need to be printed, even for signing, as by integrating the electronic signature with legal effect, the entire enterprise process becomes digitalized from beginning to end.
IDMS is a robust, modular document management product line. It includes the following modules:
- Document Storage module: It enables the structured storage of documents. Its functionality in brief: version control, the same document in multiple formats (e.g. Word + PDF), multi-volume documents, check-out / check-in, MS Office integration, customizable attributes, folder system, support for large files (even videos).
- Document Approval module: It supports the process of document editing – review – approval / signing. Arbitrary workflows can be developed (even with multiple editors / reviewers / approvers).
- Document Distribution module: Certain industry quality standards specify that specific types of documents (e.g. SOPs, operational instructions) should be provided to company employees in a controlled fashion. This module supports the controlled distribution and withdrawal of documents (and their versions) using the software management of distribution lists.
IDMS can be installed as a standalone system, or they can be used as a starting point for a solution adapted to specific needs as well.
The licensing costs of the IDMS system is substantially more favorable compared to the licensing costs of widespread enterprise document management systems (e.g. Documentum, FileNet).
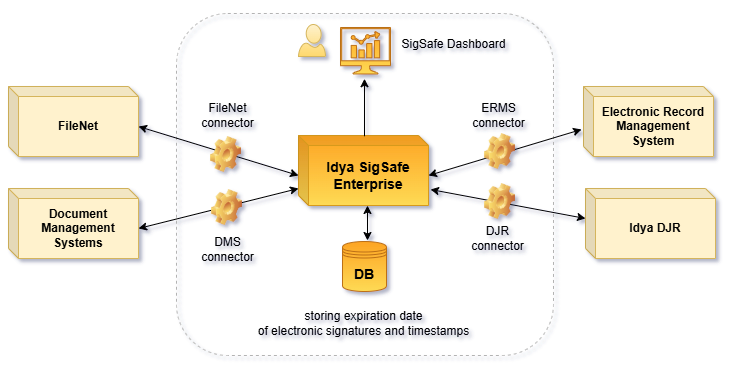
The SigSafe Enterprise supports the long-term retention of documents within the enterprise. The solution provides the verification of document authenticity, and ensures that the validity of electronic signatures is maintained (resealing), which is performed via the recurrent timestamping of documents.
For each document, SigSafe determines and stores the period of validity of the timestamps (or electronic signatures) it contains. Based on this, it continuously reseals documents in an automated, optimized manner.
SigSafe is capable of integrating with all document management systems that store electronically signed documents (e.g. FileNet, Idya DJR, electronic record management system, etc.). This allows for the centralized management of the resealing process.
The solution displays data related to the resealing process, such as the number of resealings due in the next period, on a central dashboard interface, so the transaction costs associated to this can be calculated.